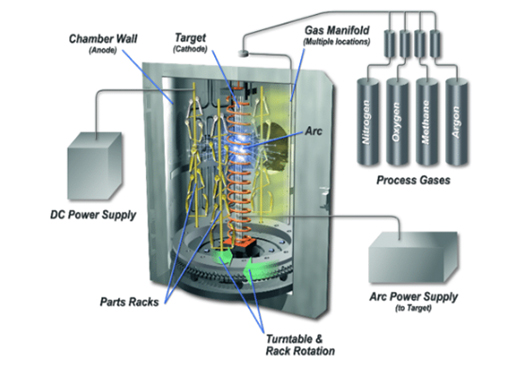
Anodizing is one of the most common metal surface treatment operations performed on aluminum parts. It is an electrochemical process that involves immersing aluminum parts in a series of tanks to transform the aluminum surface into a durable and corrosion-resistant finish.
To determine if anodizing is the right choice for a particular part, product designers must first understand how it affects the strength, thickness, color and thermal conductivity of aluminum.
This article provides answers to five common questions about anodized aluminum. If you are looking to implement anodizing in your machined products
Anodizing can usually be categorized into three types:
Type I Anodizing
Type II Anodizing
Type III Anodizing
Also known as chromic acid anodizing, a chromic acid chemical bath is used to form a coating (or oxide layer) on the aluminum surface. It produces thin coatings (up to 2.5 microns) and is ideal for applications requiring minimal corrosion protection and paint adhesion.
Uses a sulfuric acid chemical bath to form an oxide layer on aluminum parts. This type of anodizing produces an oxide layer up to 25 microns thick, making it more resistant to corrosion than Type I anodized aluminum parts. In addition, because they have a thicker oxide layer (and pores), they retain dyes and coloration better than "Type I" anodized parts.
Also known as hard coat anodizing, this process produces an oxide layer thicker than 25 microns. It uses sulfuric acid as the chemical bath, as in Type II anodizing. However, the current flows for a longer period of time in this process than in Type II anodizing. This allows them to produce thicker layers and makes them more resistant to corrosion than Type I and Type II anodized parts.

When you expose regular aluminum parts to the atmosphere, a layer of aluminum oxide forms on the surface of the part. However, this layer is usually very thin and wears off easily, especially if you scratch it or use it in places where the air is polluted.
Unlike regular aluminum, however, the oxide layer in anodized aluminum parts is located deep within the aluminum substrate. For example, the pores (and honeycomb oxide layer) formed during the electrochemical reaction can be up to 25 microns. As a result, you will have an aluminum part that is corrosion and scratch resistant and can withstand virtually any chemical attack.
The anodizing process involves immersing and treating clean aluminum parts in an electrolyte chemical bath. This chemical bath is usually made of sulfuric acid or chromic acid (a conductive solution).
Next, a direct current is applied to this chemical bath, creating a positive charge on the aluminum part and a negative charge in the electrolyte plates. The resulting electrochemical reaction creates pores on the surface of the part. These pores combine with the negatively charged O 2 ions of the electrolyte to form a honeycomb oxide layer (aluminum oxide) on the component.
Anodizing does not make aluminum parts stronger or weaker. Instead, it increases the hardness of the aluminum ㅡ, which describes the resistance of an aluminum part to surface indentations, scratches, or abrasion. For example, an anodized aluminum part may be three times as hard as the original aluminum alloy.
In addition, anodized aluminum parts are typically lighter than other metals such as copper and stainless steel. This unique property makes them ideal for aerospace applications that require a lightweight metal.
Thermal conductivity describes the ability of a material to transfer or conduct heat. This ability increases with heat flow, material thickness, and material surface area.
Because anodizing creates an additional oxide layer on the surface of an aluminum part, you will agree that it will increase the thickness and surface area of the part. As a result, anodized aluminum will have higher thermal conductivity than unfinished aluminum parts. This makes anodized aluminum parts ideal for heat sink applications in today's electronics and other thermal systems.
When choosing the right anodized finish for your application, be sure to consider your product needs as well as the desired performance characteristics. Whichever type of anodizing your project requires - Type I, Type II, or Type III - can make your aluminum alloy parts more corrosion-resistant, robust, and available in a variety of optional colors. At the same time, the thermal conductivity of aluminum alloys is critical for some applications, and anodizing can also help increase the material's ability to conduct heat.
To ensure the success of your project, we recommend that you choose an experienced company to perform anodizing and other surface treatment processes. Founded in 2008, Richconn is a professional rapid prototyping company that offers anodizing services, surface finishing services, and plating services for a variety of materials. No matter what your needs are, we will provide you with superior technology and service to ensure that your products are the best they can be in terms of performance and appearance. We look forward to working with you to add color to your projects and inject more value and competitiveness into your products.
 What is a Machined Boss: A Comprehensive GuideNovember 8, 2023In the world of precision engineering, the term machined boss may not be a household name, but it is undoubtedly a crucial element that underpins countless industries. Whether you're a novice looking to expand your knowledge or a seasoned professional seeking in-depth insights, this article will serve as your comprehensive guide to understanding what a machined boss is, how it works, and why it matters.view
What is a Machined Boss: A Comprehensive GuideNovember 8, 2023In the world of precision engineering, the term machined boss may not be a household name, but it is undoubtedly a crucial element that underpins countless industries. Whether you're a novice looking to expand your knowledge or a seasoned professional seeking in-depth insights, this article will serve as your comprehensive guide to understanding what a machined boss is, how it works, and why it matters.view 5 Important Facts About 5 Axis Machining | Basics Information, Benefits, Limitations, Applications & TipsFebruary 20, 20245 axis CNC machining technology is important in manufacturing. Learn more about its basics, pros &cons, applications, and tips to enhance your project performance.view
5 Important Facts About 5 Axis Machining | Basics Information, Benefits, Limitations, Applications & TipsFebruary 20, 20245 axis CNC machining technology is important in manufacturing. Learn more about its basics, pros &cons, applications, and tips to enhance your project performance.view Cast Aluminum vs Machined Aluminum: Unveiling the Crafting MarvelsNovember 13, 2023In the realm of metal fabrication, the choice between cast aluminum and machined aluminum holds the key to unlocking a world of possibilities. As a CNC machining service provider, we, at Richconn, understand the importance of making informed decisions in the manufacturing process. Let's embark on a journey to explore the nuances of these two techniques, dissecting their processes, comparing their performance, and uncovering their diverse applications.view
Cast Aluminum vs Machined Aluminum: Unveiling the Crafting MarvelsNovember 13, 2023In the realm of metal fabrication, the choice between cast aluminum and machined aluminum holds the key to unlocking a world of possibilities. As a CNC machining service provider, we, at Richconn, understand the importance of making informed decisions in the manufacturing process. Let's embark on a journey to explore the nuances of these two techniques, dissecting their processes, comparing their performance, and uncovering their diverse applications.view Thriving Outsourcing PartnershipsOctober 12, 2023Manufacturers now view outsourcing not just as a supplier relationship, but as a partnership. Lei Sheng, product manager at Richconn, explains why outsourcing projects with reliable partners make sense.view
Thriving Outsourcing PartnershipsOctober 12, 2023Manufacturers now view outsourcing not just as a supplier relationship, but as a partnership. Lei Sheng, product manager at Richconn, explains why outsourcing projects with reliable partners make sense.view Mastering Complex Features in Machined PartsOctober 16, 2023CNC machines are gaining in capability every year. Lathes with powered tools, cut a variety of shapes and drill non-axial or radial holes. In the past, this would have required a trip to the milling department. Machining centers are equipped with indexing heads that support "3+2" axis machining, where multiple sides of a part can be completed in just one operation. This is something all designers and engineers can be happy about.view
Mastering Complex Features in Machined PartsOctober 16, 2023CNC machines are gaining in capability every year. Lathes with powered tools, cut a variety of shapes and drill non-axial or radial holes. In the past, this would have required a trip to the milling department. Machining centers are equipped with indexing heads that support "3+2" axis machining, where multiple sides of a part can be completed in just one operation. This is something all designers and engineers can be happy about.view The Science of Black: Understanding the Chemistry Behind Surface TreatmentsDecember 4, 2023Black as a color has always captivated and fascinated humans. It is mysterious, elegant, and striking, making it a popular choice for various applications. From fashion and design to technology and en...view
The Science of Black: Understanding the Chemistry Behind Surface TreatmentsDecember 4, 2023Black as a color has always captivated and fascinated humans. It is mysterious, elegant, and striking, making it a popular choice for various applications. From fashion and design to technology and en...view
 EN
EN
 ru
ru