Passivation is a technique used in cnc machining services for the surface treatment of metals to improve their corrosion resistance, extend their service life, and enhance their appearance. The passivation process involves covering or altering the surface of a metal to a layer of compounds that are less susceptible to corrosion, and is usually accomplished by immersing the metal in a chemical treatment solution. This technique is commonly used to treat metals such as iron, aluminum, magnesium, zinc, and stainless steel to protect them from corrosion and oxidation in the environment.

The basic principle of the passivation process is the formation of a thin film or film layer on the surface of the metal which has the following properties:
Corrosion resistance: The film protects the metal surface from corrosive substances such as water, oxygen, acids, alkalis etc.
Enhanced adhesion: the film and the metal surface combined tightly, not easy to fall off or peel.
Improved electrical conductivity: A good passivation film should not hinder the conduction of electric current, which is very important in some applications, such as the manufacture of electronic equipment.
Improved wear resistance: Passivation also increases the wear resistance of the metal and extends its service life.
Aesthetics: Passivation can improve the appearance of a metal surface, making it look more attractive.
To achieve these properties, the passivation process usually involves immersing the metal part in a solution containing a specific chemical substance, which causes the metal surface to react with the components of the solution, thereby forming a thin film with the desired properties on the metal surface.
Passivation processes can be categorized into various types, depending on the chemicals used and the conditions of the process. The following are some of the common types of passivation processes:
Electroplating: This is a method of passivation achieved by electrochemically depositing a film of a metal or alloy on a metal surface. Examples include chrome plating, galvanizing, nickel plating, etc. These coatings not only provide corrosion resistance but also improve appearance.
Phosphating: Phosphating is the process of converting a metal surface into a phosphorus compound. Phosphate coatings provide excellent corrosion resistance and are commonly used for surface treatment of steel parts, such as automotive parts.
Anodizing: This is the process of electrochemically oxidizing a metal surface to an oxide, usually used for aluminum. The oxidized aluminum surface usually has better corrosion and wear resistance.
Chemical Conversion Coating: This type of passivation process involves reacting the metal surface with chemicals to form a chemical conversion layer. Phosphating, chromate passivation and aluminum passivation are all common types of chemical conversion coatings.
Passivation of Stainless Steel: Stainless steel often contains alloying elements other than iron, so rust can form during fabrication and welding. Passivation of Stainless Steel is a process that removes rust and other impurities from the surface of stainless steel and forms a protective film.
Electrochemical passivation (Electropolishing): This is a process of electrochemically removing small inhomogeneities from a metal surface, making it smoother, more corrosion resistant and improving its appearance.
Passivation has a wide range of applications in various fields, some of the major areas are listed below:
Automotive Industry : Passivation processes are used in the manufacture of automotive parts to provide corrosion resistance and extend the life of the parts. This includes galvanizing, phosphating and coating processes.
Electronic Industry: Circuit boards and components in electronic devices often require good electrical conductivity, so oxidation and other passivation processes are used to improve the performance of these metals.
Aerospace industry: Aerospace and aviation components require a high degree of corrosion and temperature resistance, so stainless steel and other passivation processes are important in these applications.
Construction: Passivation processes are used in the manufacture and treatment of construction materials such as steel structures, aluminum windows and doors to improve their weathering resistance.
Food and Pharmaceutical Industry: In equipment and containers that come into contact with food and pharmaceuticals, stainless steel passivation is used to ensure the purity of the product and to prevent metallic impurities from entering the product.
Offshore: Due to the corrosive nature of seawater, metals used in offshore engineering often require additional corrosion protection, and passivation plays a key role in this regard.
Chemical and Petroleum Industry: In these fields, vessels and pipes handling corrosive chemicals require corrosion protection and hence various passivation processes are used.
Passivation is extremely important in modern industry for the following reasons:
Extended service life: Passivation can greatly extend the service life of metal products, thereby reducing the cost of equipment replacement and maintenance.
Reduced Maintenance Costs: By providing corrosion resistance, passivation reduces the need for maintenance of equipment and structures, reducing repair costs.
Improved performance: Passivation processes can improve the performance of metals, such as wear resistance, electrical conductivity and thermal conductivity, making them more viable for a variety of applications.
Environmentally Friendly: Passivation is often more environmentally friendly than other surface treatments because it reduces reliance on harmful chemicals while minimizing the generation of waste and pollutants.
Ensure Product Quality: In areas such as food, pharmaceuticals and electronics, passivation ensures product purity and quality to meet stringent industry standards.
Improve appearance: Passivation can also improve the appearance of a product, making it more attractive for applications that require a high degree of finishing.
Passivation processes play a key role in several industrial sectors, not only improving product performance and quality, but also reducing costs and helping to maintain sustainable production and manufacturing processes. As technology continues to advance, passivation processes will continue to evolve to meet growing industrial demands and environmental standards.

Galvanization is a common method of metal passivation used primarily to protect iron or steel surfaces from corrosion. In galvanization, iron or steel is immersed in molten zinc containing zinc ions and an electric current is passed through it. This causes the zinc ions to reduce and deposit on the surface of the iron or steel, forming a strong zinc coating. This zinc coating is an effective barrier to water and oxygen, thus extending the life of the iron or steel product.
Aluminum Anodizing is a metal passivation method used to treat aluminum surfaces. In this process, aluminum pieces are immersed in a solution containing an acidic electrolyte and then oxidized by passing an electric current through them. This creates an aluminum oxide film on the surface of the aluminum, which provides excellent corrosion resistance and hardness. Aluminum oxide films can also be tinted in colors to meet different aesthetic and marking needs.
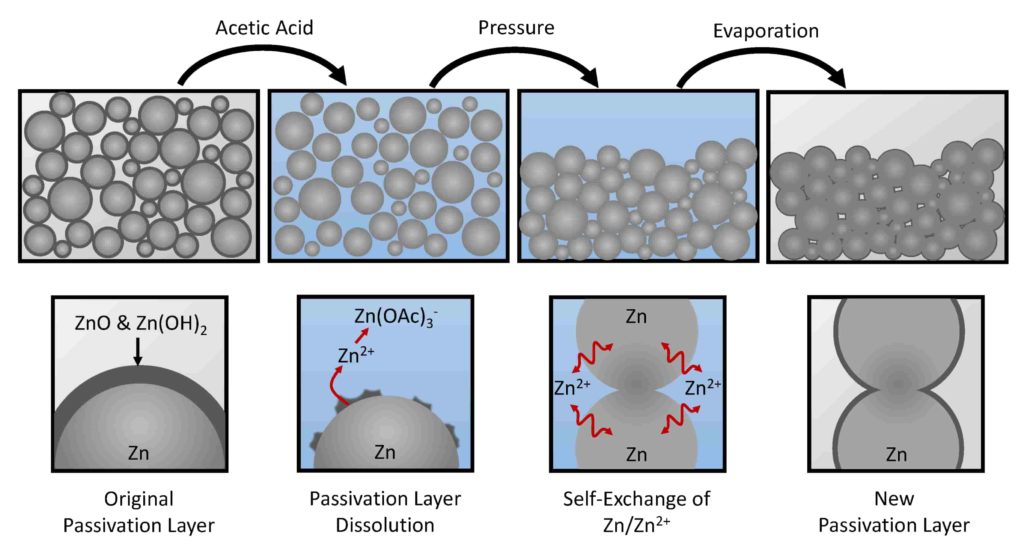
Stainless steel is a metal with good corrosion resistance, but rust and other impurities may form on the surface during the manufacturing process. In order to remove these impurities and form a protective oxide film, stainless steel passivation is often employed. This involves immersing stainless steel parts in a solution containing acids and oxidizing agents to remove impurities and induce the formation of an oxide film.
Phosphating is a method used to improve the corrosion resistance of steel surfaces. In the phosphating process, steel parts are immersed in an acidic solution containing phosphates to form a layer of phosphorus compounds on the surface. This phosphating layer provides good corrosion resistance and makes steel products more durable.

Passivation chemicals can be categorized into several main types, each suitable for different types of metals and applications. Here are some common types of passivation chemicals:
Acid-based Passivation: This type of passivate usually contains an acidic component such as nitric, phosphoric, sulfuric or hydrochloric acid. They are primarily used for stainless steel, nickel alloys and other corrosion resistant metals. Acidic passivates remove impurities from the surface of the metal and form an oxide film on the surface of the metal to improve corrosion resistance.
Alkaline-based Passivation: These passivates contain an alkaline component such as sodium hydroxide or potassium hydroxide. Alkaline-based passivates are commonly used on metals such as iron, steel and aluminum. They remove surface oxides, remove impurities and form a protective oxide film.
Chromate-based Passivation: Chromate-based passivates contain hexavalent chromium or trivalent chromium compounds such as hexavalent chromic acid or trivalent chromium chloride. These chemicals are mainly used for passivation of aluminum and magnesium, as well as for sealing of some coatings. The films they form usually have good corrosion resistance and electrical conductivity.
Iron-Based Passivation: Iron-based passivation fluids are commonly used for the treatment of steel and iron to improve their corrosion resistance. These fluids contain some iron compounds such as ferricyanide.
Zinc-based Passivation: Zinc-based passivation fluids are typically used for the treatment of galvanized steel and galvanized iron to provide additional corrosion protection. These fluids contain zinc salts or other zinc compounds.
Titanium-based Passivation: Titanium-based passivation fluids are used for the treatment of titanium and titanium alloys. These fluids usually contain some titanium compounds such as titanates.
Conversion Coating: These passivation fluids usually contain a variety of ingredients such as acids, bases and metal salts. They are used to produce surface conversion coatings such as phosphating, chromate passivation and zinc-magnesium phosphate coatings.
Now, let's take a more detailed look at some common passivation chemicals and their applications in metal passivation:
Chemical composition: Hexavalent chromates are compounds containing hexavalent chromium, such as potassium hexavalent chromate and ammonium hexavalent chromate.
Applications: Hexavalent chromates are widely used for passivation of stainless steel and aluminum. They form oxide films with excellent corrosion resistance and are used in food processing equipment, medical devices and aerospace components.
Environmental and Health Considerations: Hexavalent chromates are considered harmful to the environment and health because hexavalent chromium is a potential carcinogen. Therefore, research on alternatives is ongoing.
Chemical Composition: Nitric acid is an acidic passivate, usually in the form of concentrated nitric acid.
Applications: Concentrated nitric acid is commonly used in the acid passivation of stainless steel. It removes impurities from the metal surface and encourages the formation of an oxide film.
Environmental and Health Considerations: Concentrated nitric acid is a strong acid and must be handled with care. Its waste also needs to be disposed of in accordance with the relevant regulations.
Chemical composition: Sodium hydroxide is an alkaline passivate, usually in the form of a sodium hydroxide solution.
Applications: Sodium hydroxide is commonly used in the passivation of iron and steel to remove oxides and form a protective oxide film. It is also used for passivation of aluminum.
Environmental and Health Considerations: Sodium hydroxide is a corrosive chemical and requires careful use. Waste sodium hydroxide solutions need to be disposed of properly.
Chemical composition: Phosphates are a group of compounds that include phosphoric acid and some metal salts such as sodium ferrocyanide.
Applications: Phosphates are commonly used for passivation of iron and steel to provide corrosion resistance. Sodium ferrocyanide is also used for blue passivation of iron and steel.
Environmental and Health Considerations: Sodium ferrocyanide is a relatively safe chemical, but caution is still required in its use and disposal.
Chemical composition: Zinc compounds include zinc salts and other compounds containing zinc.
Applications: Zinc compounds are commonly used in the treatment of galvanized steel to provide additional corrosion protection. They are also used for passivation of some aluminum alloys.
Environmental and Health Considerations: Zinc compounds are generally relatively safe, but waste needs to be disposed of properly to prevent environmental contamination.
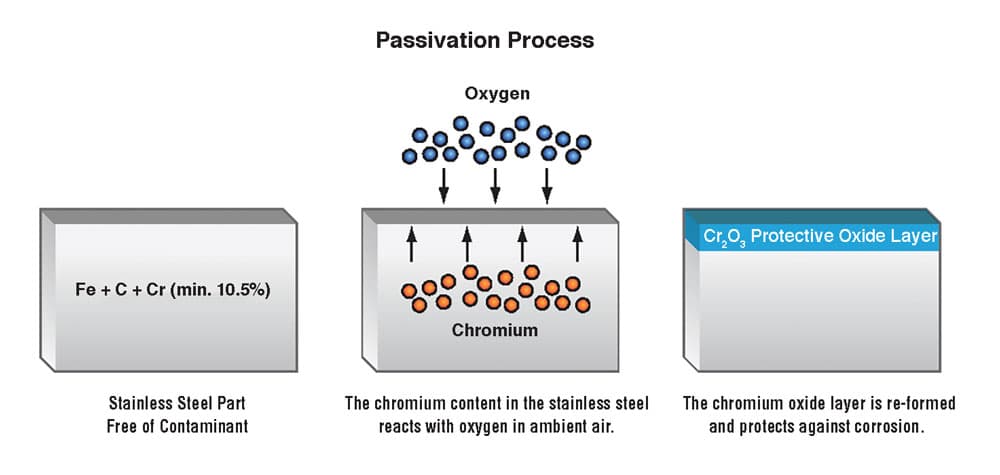
Stainless steel passivation is an important surface treatment process used to improve the corrosion resistance and extend the service life of stainless steel materials. Stainless steel itself has excellent corrosion resistance, but under some specific conditions, corrosion problems may still occur. With stainless steel passivation, the corrosion resistance of stainless steel can be further improved, making it more reliable in harsh environments.
Stainless steel passivation is a process that improves the corrosion resistance of stainless steel by improving the chromium oxide layer on its surface. The principle of this process involves the following aspects:
Improvement of the chromium oxide layer: The corrosion resistance of stainless steel depends primarily on the chromium oxide layer on the surface. One of the goals of passivation of stainless steel is to increase the stability and homogeneity of the chromium oxide layer. This can be achieved by removing impurities and defects from the surface and by promoting the regeneration of the chromium oxide layer.
Elimination of reactive parts: Stainless steel surfaces may have reactive parts that are particularly sensitive to corrosion. The process of passivation of stainless steel helps to eliminate these active portions, thereby reducing the risk of corrosion.
Improved resistance to chloride corrosion: Chloride is a common source of corrosion that poses a threat to the corrosion performance of stainless steel. Passivation of stainless steel improves its resistance to chlorides, thereby extending its service life.
Improved Acid Resistance: Stainless steel can also be corroded in some acidic environments. Through stainless steel passivation, its acid resistance can be improved, making it suitable for a wider range of applications.
There are various methods of passivation of stainless steel, and the choice of method usually depends on the requirements of the specific application and environmental conditions. The following are some common stainless steel passivation methods:
Acid Stainless Steel Passivation: Acid stainless steel passivation fluids typically include nitric acid or nitrates, which remove contaminants and oxides from the surface of the stainless steel and induce the formation of a chromium oxide layer. The process is usually carried out in a dipping or spraying environment.
Alkaline Stainless Steel Passivation: Alkaline stainless steel passivation fluids include sodium hydroxide or potassium hydroxide, which are typically used to remove oxides from the surface of stainless steel and enhance the stability of the chromium oxide layer. This method can also be carried out by dipping or spraying.
Chromium-containing stainless steel passivation: Chromium-containing stainless steel passivation uses chemicals containing hexavalent chromium, such as hexavalent chromates. These chemicals help to improve the stability of the chromium oxide layer, making it more resistant to corrosion. This method is typically used in highly corrosion resistant environments.
Electrochemical Stainless Steel Passivation: Electrochemical stainless steel passivation is a method of controlling the formation of a chromium oxide layer on the surface of stainless steel by passing an electric current through it. This method is useful in some specialized applications because it provides finer control.
Phosphate Stainless Steel Passivation: Phosphate stainless steel passivation involves immersing the surface of the stainless steel in an acidic liquid containing phosphates to induce the formation of phosphorus compounds. This method is commonly used to improve the wear and corrosion resistance of stainless steel.
Nitrided Stainless Steel Passivation: Nitrided stainless steel passivation is a method of penetrating nitrogen atoms into the surface of stainless steel by injecting nitrogen into a high-temperature, high-pressure environment. This increases the hardness and corrosion resistance of the stainless steel.
Passivation processes play a key role in several industrial sectors, not only improving product performance and quality, but also reducing costs and helping to maintain sustainable production and manufacturing processes. As technology continues to advance, passivation processes will continue to evolve to meet growing industrial demands and environmental standards.
 Metal Stamping Die: Professional, Speed, AdvantagesApril 25, 2023Mold products are needed in many industries. Screws and nuts that we use in our daily life, pins and patches used in the electronics industry, equipment housing and internal parts used in the medical ...view
Metal Stamping Die: Professional, Speed, AdvantagesApril 25, 2023Mold products are needed in many industries. Screws and nuts that we use in our daily life, pins and patches used in the electronics industry, equipment housing and internal parts used in the medical ...view CNC Machining and AI: How Artificial Intelligence is Impacting the FieldOctober 25, 2023Artificial Intelligence is having a profound impact on CNC machining services, transforming operations and delivering many benefits. Here are some of the key areas where AI is playing a major role:view
CNC Machining and AI: How Artificial Intelligence is Impacting the FieldOctober 25, 2023Artificial Intelligence is having a profound impact on CNC machining services, transforming operations and delivering many benefits. Here are some of the key areas where AI is playing a major role:view Titanium vs. Aluminum: Which is the Best Lightweight Metal?November 23, 2023Titanium and Aluminum are two excellent lightweight metals, and when it comes to machining, the choice of material is a crucial decision. Titanium and Aluminum are both common lightweight metal materials, and both have their own unique advantages in different situations.view
Titanium vs. Aluminum: Which is the Best Lightweight Metal?November 23, 2023Titanium and Aluminum are two excellent lightweight metals, and when it comes to machining, the choice of material is a crucial decision. Titanium and Aluminum are both common lightweight metal materials, and both have their own unique advantages in different situations.view Spray painting, powder spraying, electrophoresis: three common surface treatment methodsMarch 6, 2024Spray painting is a common way of surface treatment, its principle is to use air pressure to spray paint from the nozzle, forming tiny droplets, that and evenly attached to the surface of the painted object.view
Spray painting, powder spraying, electrophoresis: three common surface treatment methodsMarch 6, 2024Spray painting is a common way of surface treatment, its principle is to use air pressure to spray paint from the nozzle, forming tiny droplets, that and evenly attached to the surface of the painted object.view Waterjet Cutting: Methods, Applications, and BenefitsJune 21, 2024Waterjet cutting is a special cutting technique that is progressively drawing interest from an increasing number of industries. The technical underpinnings, benefits, and areas of application of waterjet cutting will be thoroughly covered in this article.view
Waterjet Cutting: Methods, Applications, and BenefitsJune 21, 2024Waterjet cutting is a special cutting technique that is progressively drawing interest from an increasing number of industries. The technical underpinnings, benefits, and areas of application of waterjet cutting will be thoroughly covered in this article.view Exploring Chameleon PVD Coating in DesignJanuary 5, 2024The world of design is constantly evolving, with new techniques and materials pushing boundaries and inspiring creativity. One such innovation that has revolutionized the design industry is Chameleon ...view
Exploring Chameleon PVD Coating in DesignJanuary 5, 2024The world of design is constantly evolving, with new techniques and materials pushing boundaries and inspiring creativity. One such innovation that has revolutionized the design industry is Chameleon ...view